Process Development For Viral Vector Manufacturing

Viral Vector Process Development And Manufacturing Summit Idt Biologika Elevate your viral vector process development & manufacturing in boston 2024. join 150 experts, streamline quality, and cut costs!. Optimize the viral vector development process, figure 3, ensuring it is repeatable, scalable, and transferable to cgmp manufacturing. to ensure efficient scale up, increase titers, reduce development timelines, and lower costs to support upstream and downstream process development.
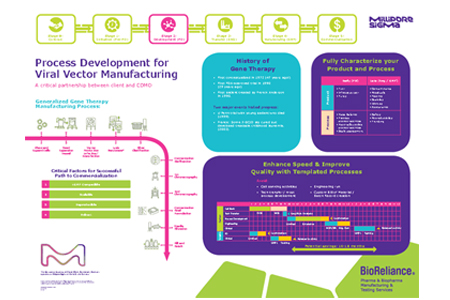
Process Development For Viral Vector Manufacturing Process development for a viral vector involves optimizing and scaling up the production process to efficiently and consistently generate high quality viral vectors for use in gene therapy, vaccines, and other applications. learn how we do that at batavia biosciences. Producing viral vectors in cell lines grown in bioreactors instead of conventional 2d cultivation systems allows for close process monitoring and control , simplifies bioprocess scale up , and as a result opens up new possibilities for bioprocess optimization to increase yield and reproducibility. At its core, viral vector manufacturing revolves around selecting suitable producer cell lines (frequently mammalian cells), optimizing the production process, and adhering to strict regulatory standards as well as current good manufacturing practices (cgmp). Read about the benefits and drawbacks of transient transfection and different scale up methods for upstream bioprocessing for manufacturing viral vectors. viral vectors are fascinating tools that the biopharmaceutical industry is using to deliver genetic material for therapeutic application.

Viral Vector Process Development Summit Somatic Cell Gene Editing At its core, viral vector manufacturing revolves around selecting suitable producer cell lines (frequently mammalian cells), optimizing the production process, and adhering to strict regulatory standards as well as current good manufacturing practices (cgmp). Read about the benefits and drawbacks of transient transfection and different scale up methods for upstream bioprocessing for manufacturing viral vectors. viral vectors are fascinating tools that the biopharmaceutical industry is using to deliver genetic material for therapeutic application. Viral vector design involves inserting custom genes into viral vectors, which promote the expression of therapeutic or functional proteins in the host. from this perspective, viral vectors are powerful delivery vehicles for gene therapies, correcting deficiencies in genetic disorders, such as muscular atrophy. Process development plays a critical role in viral vector manufacturing as it establishes and optimizes suitable cell culture and purification methods, and develops analytical assays for product quality confirmation. Our deep technical knowledge is allied with more than 20 years of regulatory and drug development experience to ensure we begin vector development with a successful end in mind. this, coupled with our viral vector manufacturing platform and plasmid capabilities, means we are a one stop shop for vector production by transfection. Industry leading platform enables process development and cgmp manufacturing of viral vector drug product under 6 months. we offer a purpose built facility specifically designed for viral vector manufacturing. our processes adhere to stringent global regulatory guidelines, including fda, eu, and nmpa standards.

Viral Vector Manufacturing Process Viral vector design involves inserting custom genes into viral vectors, which promote the expression of therapeutic or functional proteins in the host. from this perspective, viral vectors are powerful delivery vehicles for gene therapies, correcting deficiencies in genetic disorders, such as muscular atrophy. Process development plays a critical role in viral vector manufacturing as it establishes and optimizes suitable cell culture and purification methods, and develops analytical assays for product quality confirmation. Our deep technical knowledge is allied with more than 20 years of regulatory and drug development experience to ensure we begin vector development with a successful end in mind. this, coupled with our viral vector manufacturing platform and plasmid capabilities, means we are a one stop shop for vector production by transfection. Industry leading platform enables process development and cgmp manufacturing of viral vector drug product under 6 months. we offer a purpose built facility specifically designed for viral vector manufacturing. our processes adhere to stringent global regulatory guidelines, including fda, eu, and nmpa standards.

Viral Vector Manufacturing Process Our deep technical knowledge is allied with more than 20 years of regulatory and drug development experience to ensure we begin vector development with a successful end in mind. this, coupled with our viral vector manufacturing platform and plasmid capabilities, means we are a one stop shop for vector production by transfection. Industry leading platform enables process development and cgmp manufacturing of viral vector drug product under 6 months. we offer a purpose built facility specifically designed for viral vector manufacturing. our processes adhere to stringent global regulatory guidelines, including fda, eu, and nmpa standards.
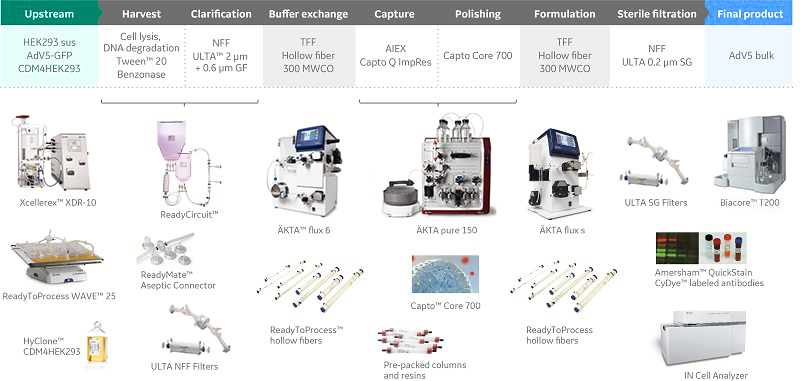
Process Development For Viral Vector Manufacturing Vrogue Co

Comments are closed.