Viral Vector Gene Therapy Study Using Aav Based Vectors Lays Foundation
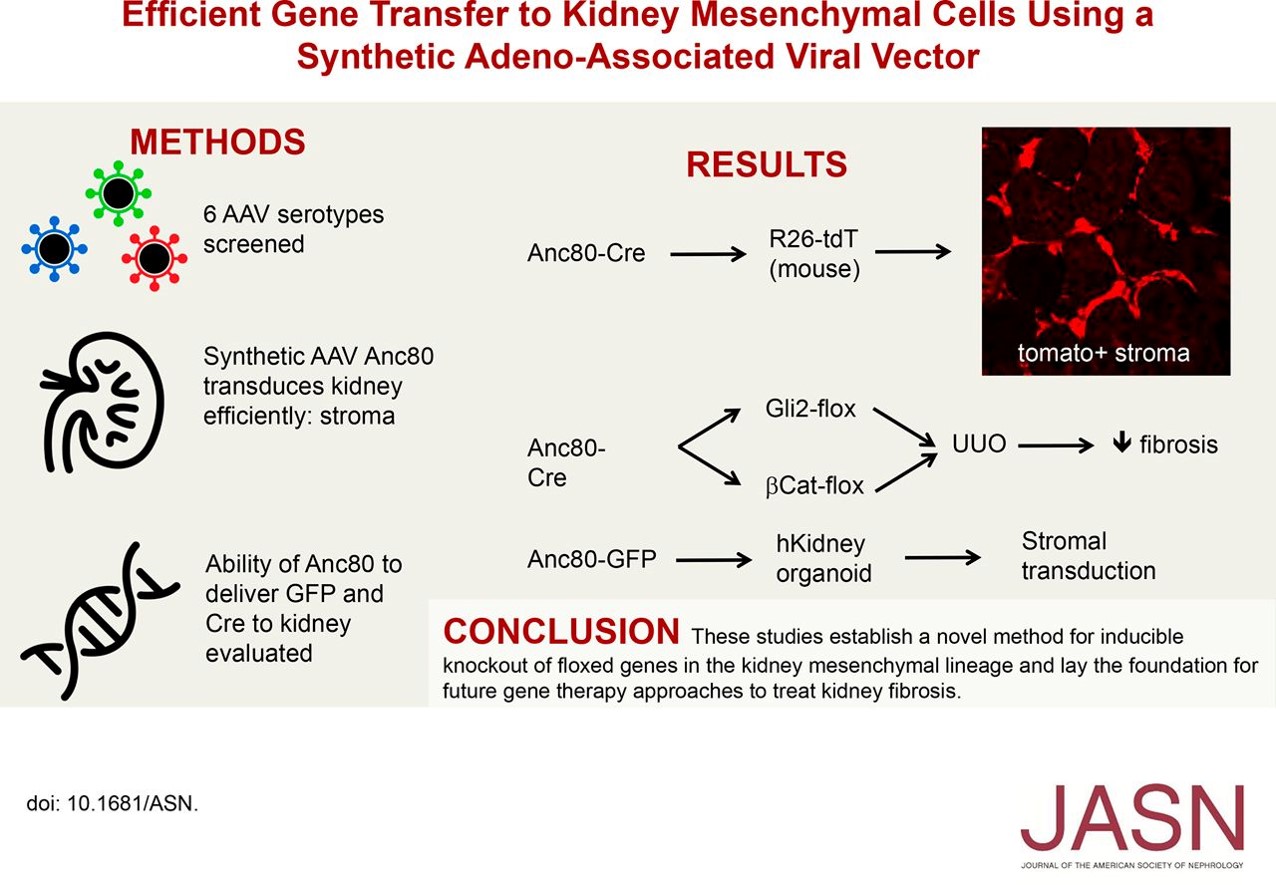
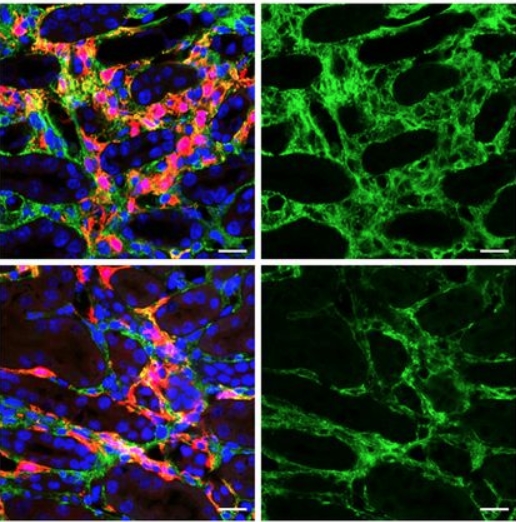
Gene Therapy Study Using Aav Based Vectors Lays Foundation For Kidney A new study headed by researchers in the humphreys laboratory in the division of nephrology at washington university, however, is laying the groundwork for the use of adeno associated virus (aav) vectors to inhibit renal fibrosis via targeted gene therapy. their “targets” are the mesenchymal progenitors in the kidney interstitium that. Here, we sought to optimize a novel, cpg rich aav8 vector, referred to as pvr59, designed for treating lipoprotein lipase deficiency (lpld). we strategically reduced cpg levels in pvr59,.
Gene Therapy Study Using Aav Based Vectors Lays Foundation For Kidney Viral based gene therapy utilizes viruses as gene delivery vectors, including aav, adenovirus, retrovirus, lentivirus, and herpes simplex virus. non viral based gene therapy includes. Adeno associated virus (aav) is a versatile viral vector technology that can be engineered for very specific functionality in gene therapy applications. to date, aav has been shown to be safe and effective in preclinical and clinical settings. Using viral vector systems for gene therapy are promising treatment options for several diseases. however, these methods remain risky and are still under study to ensure safety and efficacy during clinical trials. Develop and make broadly available a robust, economically viable, shared access platform for the technical development, manufacturing, and characterization of aav based gene therapy vectors.

Gene Therapy Study Using Aav Based Vectors Lays Foundation For Kidney Using viral vector systems for gene therapy are promising treatment options for several diseases. however, these methods remain risky and are still under study to ensure safety and efficacy during clinical trials. Develop and make broadly available a robust, economically viable, shared access platform for the technical development, manufacturing, and characterization of aav based gene therapy vectors. The fda has approved the gene therapy products based on two viral vectors, which are both aav vectors: luxturna (spark therapeutics, inc.) for the treatment of patients with confirmed biallelic rpe65 mutation associated retinal dystrophy and zolgensma for the treatment of pediatric patients below two years of age having spinal muscular atrophy. This study lays the foundation for continued development of immunogenicity of delivery vectors such as adeno associated virus in patients who have been previously exposed to the aav capsid as part of a common cold infection or previous aav gene therapy treatment. 44, 45 non viral vectors such as lnp suffer the challenge of. Gene therapy with aav vectors is a promising approach for treating numerous genetic disorders but is often hindered by preexisting antibodies that neutralize the vectors. given that females may. Lentiviral and adenoviral based gene therapy vectors pose safety concerns associated with pathogenicity and vector integration. however, due to aav’s reliance on helper viruses for replication, aav provides a safer alternative, decreasing the likelihood of vector integration and pathogenicity 2.

Aav Based Gene Therapy By Vector Design A Number Of Aav Based Gene The fda has approved the gene therapy products based on two viral vectors, which are both aav vectors: luxturna (spark therapeutics, inc.) for the treatment of patients with confirmed biallelic rpe65 mutation associated retinal dystrophy and zolgensma for the treatment of pediatric patients below two years of age having spinal muscular atrophy. This study lays the foundation for continued development of immunogenicity of delivery vectors such as adeno associated virus in patients who have been previously exposed to the aav capsid as part of a common cold infection or previous aav gene therapy treatment. 44, 45 non viral vectors such as lnp suffer the challenge of. Gene therapy with aav vectors is a promising approach for treating numerous genetic disorders but is often hindered by preexisting antibodies that neutralize the vectors. given that females may. Lentiviral and adenoviral based gene therapy vectors pose safety concerns associated with pathogenicity and vector integration. however, due to aav’s reliance on helper viruses for replication, aav provides a safer alternative, decreasing the likelihood of vector integration and pathogenicity 2.

Aav Based Gene Therapy By Vector Design A Number Of Aav Based Gene Gene therapy with aav vectors is a promising approach for treating numerous genetic disorders but is often hindered by preexisting antibodies that neutralize the vectors. given that females may. Lentiviral and adenoviral based gene therapy vectors pose safety concerns associated with pathogenicity and vector integration. however, due to aav’s reliance on helper viruses for replication, aav provides a safer alternative, decreasing the likelihood of vector integration and pathogenicity 2.

Comments are closed.