Viral Vector Production Series Keeping Up With Regulatory Demands

Guide To Viral Vector Production Whitepaper Batavia Cytivalifesciences en us solutions bioprocessing products and solutions vaccine platformsin our tutorial series on viral vector production, g. We expect regulatory requirements to increase and get more standardized as viral vector based therapeutics gain greater adoption and reach larger populations. purity demands are high and increasing and are especially challenging for high dose therapies.

C94d851b38bf40b7ba4a28c20871cf24 Png Complexities in production, low yields, and stringent regulatory requirements make viral vector manufacturing a lengthy and resource intensive process. this blog explores the critical barriers to efficient viral vector production and discusses practical strategies and emerging technologies that can help optimize and streamline the development. Viral vector gene therapies are here to stay. keeping pace with increasing demand requires consideration of challenges, the potential for standardization, and strategizing for accelerating patient access. In viral vector production, the convergence of quality evidence, adherence to good manufacturing practices (gmp), and the stringent regulatory demands, e.g. by the fda, is essential for clinical trials, commercial manufacturing, and the eventual in vivo applications of viral vectors. The field of viral vector production is evolving rapidly, driven by advancements in gene and cell therapies and increasing regulatory scrutiny. as these therapies expand, regulatory agencies emphasize analytical rigor to ensure safety, efficacy, and quality.
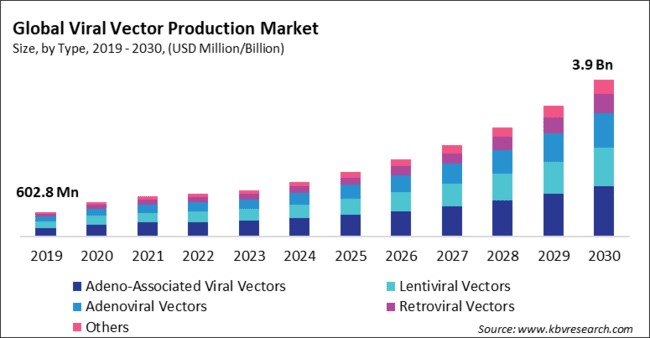
Viral Vector Production Market Size Growth Forecast 2030 In viral vector production, the convergence of quality evidence, adherence to good manufacturing practices (gmp), and the stringent regulatory demands, e.g. by the fda, is essential for clinical trials, commercial manufacturing, and the eventual in vivo applications of viral vectors. The field of viral vector production is evolving rapidly, driven by advancements in gene and cell therapies and increasing regulatory scrutiny. as these therapies expand, regulatory agencies emphasize analytical rigor to ensure safety, efficacy, and quality. Keeping up with rising demand necessitates a thorough examination of issues, the opportunity for standardization, and planning for faster patient access. gene treatments based on viral vectors have become a reality in the last five years. Learn critical facility design and process considerations that are fundamental for efficient and compliant viral vector based therapy production; gain insights into the regulatory environment, including practical advice on navigating approvals and ensuring compliance. Quality control and compliance: adhering to regulatory guidelines and implementing stringent quality control measures. by addressing these critical areas, the right cdmo partner can help clients overcome technical challenges and achieve successful outcomes in viral vector production. This article explores techniques and challenges in optimizing pharmaceutical viral vector production for gene therapy and vaccine development. topics include vector design, cell culture, purification, and quality control, along with strategies like cell line engineering, bioprocess optimization, and ai integration.
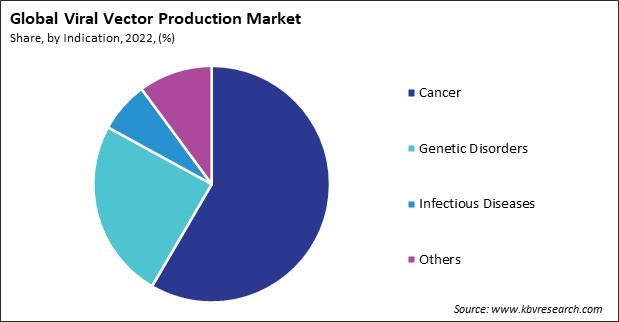
Viral Vector Production Market Size Growth Forecast 2030 Keeping up with rising demand necessitates a thorough examination of issues, the opportunity for standardization, and planning for faster patient access. gene treatments based on viral vectors have become a reality in the last five years. Learn critical facility design and process considerations that are fundamental for efficient and compliant viral vector based therapy production; gain insights into the regulatory environment, including practical advice on navigating approvals and ensuring compliance. Quality control and compliance: adhering to regulatory guidelines and implementing stringent quality control measures. by addressing these critical areas, the right cdmo partner can help clients overcome technical challenges and achieve successful outcomes in viral vector production. This article explores techniques and challenges in optimizing pharmaceutical viral vector production for gene therapy and vaccine development. topics include vector design, cell culture, purification, and quality control, along with strategies like cell line engineering, bioprocess optimization, and ai integration.
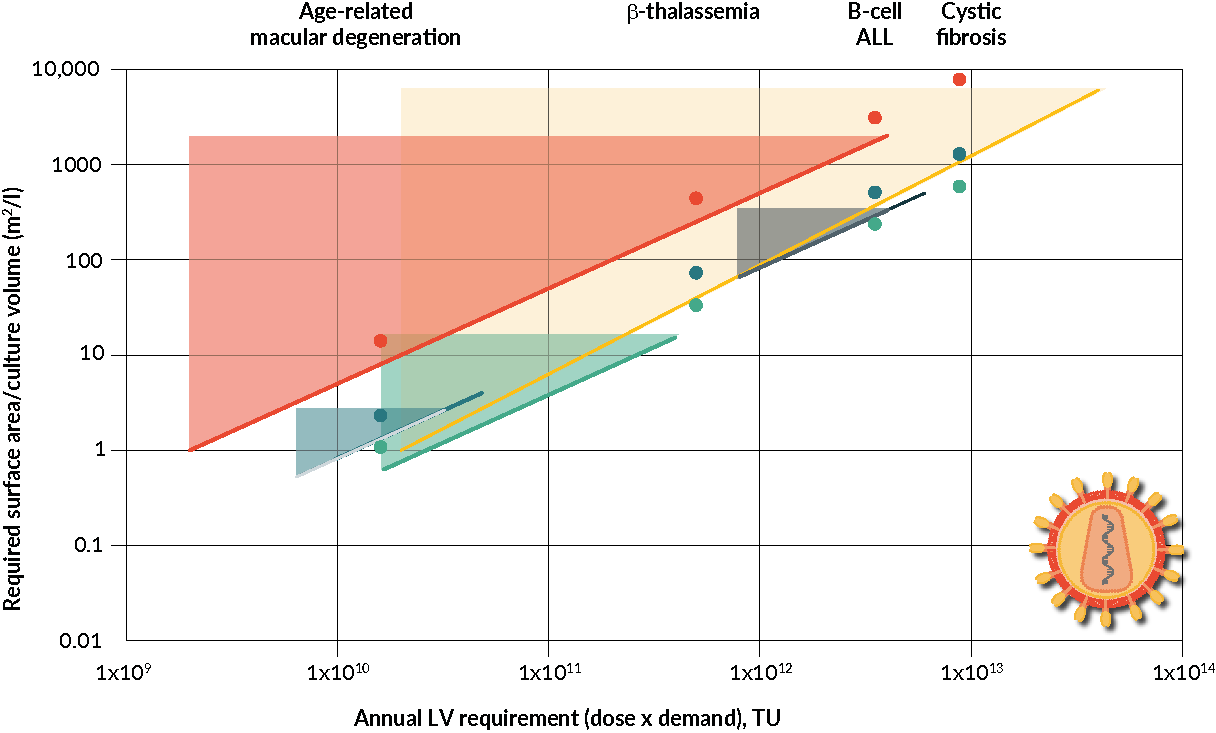
Bioinsights Viral Vector Manufacturing How To Address Current And Quality control and compliance: adhering to regulatory guidelines and implementing stringent quality control measures. by addressing these critical areas, the right cdmo partner can help clients overcome technical challenges and achieve successful outcomes in viral vector production. This article explores techniques and challenges in optimizing pharmaceutical viral vector production for gene therapy and vaccine development. topics include vector design, cell culture, purification, and quality control, along with strategies like cell line engineering, bioprocess optimization, and ai integration.
Overcoming Viral Vector Production Challenges Danaher Life Sciences

Comments are closed.