Viral Vector Production Series Scale Up Of Viral Vector Production

Viral Vector Production Unit Upv Meanwhile, sanyal points to the transition to a 3d suspension, bioreactor based process as a key first step in scaling up viral vector manufacturing. the next smart step from there is process optimization for upstream unit operations to maximize viral vector productivity per cell. Successfully scaling up viral vector production presents unique challenges that require careful consideration of both process parameters and cell line selection. at cytion, we've observed that the choice of cell line is particularly crucial for maintaining vector quality during scale up.

Viral Vector Production Unit Upv Scale up of adherent cells for vector manufacturing. industrial scale production of viral vectors is achieved by either increasing the number of culture system units (scale out) or sequential use of larger devices (scale up). The ability to seamlessly scale up and scale out the production of viral vectors is critical for meeting the increasing demand for gene therapy and cell therapy products, as well as for conducting rigorous clinical studies. Viral vectors, crucial in gene therapy scale up, differ in their integration into host cell dna—lentivirus vectors allow permanent integration, while adeno associated virus (aav) vectors are more transient. Keys to successful scale up of suspension based viral vector processes. suspension cell culture for viral vector production is highly complex, and outcomes are influenced by a multitude of factors. as such, there is no “silver bullet” for ensuring successful scale up of these processes.

Viral Vector Production Unit Upv Viral vectors, crucial in gene therapy scale up, differ in their integration into host cell dna—lentivirus vectors allow permanent integration, while adeno associated virus (aav) vectors are more transient. Keys to successful scale up of suspension based viral vector processes. suspension cell culture for viral vector production is highly complex, and outcomes are influenced by a multitude of factors. as such, there is no “silver bullet” for ensuring successful scale up of these processes. In our tutorial series on viral vector production, cytiva scientists share their insights on virus production and how to tackle viral vector manufacturing challenges. in this. The viral vector market is highly active, and the interest in production technologies is driven by recent approvals in cell and gene therapy as well as vaccines. here i share insights around the challenges in viral vector manufacturing. Scaling up viral vector production using adherent cell culture systems is challenging. learn how suspension cell culture systems benefit large scale bioprocessing.
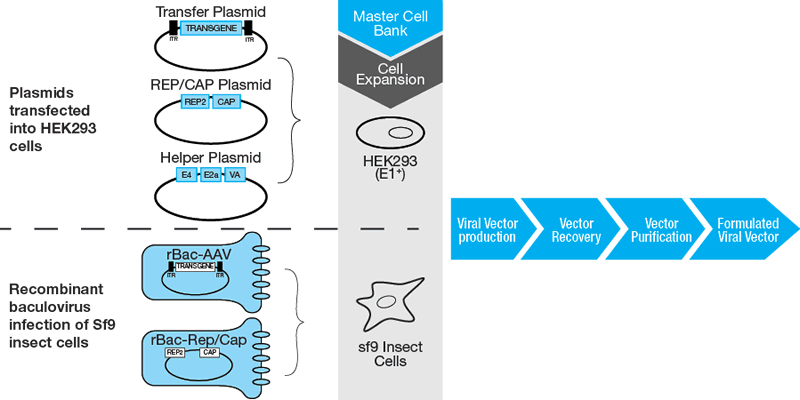
Viral Vector At Vectorified Collection Of Viral Vector Free For In our tutorial series on viral vector production, cytiva scientists share their insights on virus production and how to tackle viral vector manufacturing challenges. in this. The viral vector market is highly active, and the interest in production technologies is driven by recent approvals in cell and gene therapy as well as vaccines. here i share insights around the challenges in viral vector manufacturing. Scaling up viral vector production using adherent cell culture systems is challenging. learn how suspension cell culture systems benefit large scale bioprocessing.

Comments are closed.