Viral Vectors Quality Assistance
Viral Vectors Quality Assistance With extensive experience in the analysis of innovative products and thanks to a continuous investment in new technologies and machinery, quality assistance can assist you in the development of your recombinant adeno associated viral vectors (raavs) products from early phases to commercialisation. With 15 years expertise with (sec )mals systems and method development, this state of the art equipment adds up to our capabilities for viral vectors, biotherapeutics, vaccines and mrna based therapies analytics.
Viral Vectors Quality Assistance 12 years' experience with viral vectors. due to their complex structure and size, as well as a constantly evolving regulatory landscape, the physicochemical and biological characterisation of viral vector based products is challenging. Compared to standard qpcr, also available at quality assistance, this equipment provides a more precise and sensitive solution for a wider range of applications, such as: • detection of residual dna, • determination of the viral vector genome integrity • quantification of viral vector particles • quantification of viral load. Our key objective is to deliver high quality aav vectors to our diverse client base, including academic institutions, government bodies, foundations, and biotech industry players. learn more about the role of aav vector manufacturing in gene therapy. Adenovirus, measles, and vesicular stomatitis virus (vsv) are the most common vectors used in viral vectored vaccines given their strong immune response. replication defective adenovirus vectors are the most common viral vaccine vectors used in current investigational vaccines.

Viral Vectors Icom Creative Our key objective is to deliver high quality aav vectors to our diverse client base, including academic institutions, government bodies, foundations, and biotech industry players. learn more about the role of aav vector manufacturing in gene therapy. Adenovirus, measles, and vesicular stomatitis virus (vsv) are the most common vectors used in viral vectored vaccines given their strong immune response. replication defective adenovirus vectors are the most common viral vaccine vectors used in current investigational vaccines. With ngs biotesting, pathoquest offers characterization, quality control and release of viral vectors & plasmids across the manufacturing journey. learn more. 3pbiovian offers comprehensive access to quality control analytics for both adenovirus and aav vector gmp batches, ensuring compliance with regulatory standards for human use. our services include compendial assays, sample specific validation, and assistance in developing and validating product specific analytical methods. Companies developing viral vectors should seek outsourcing partners that can help reduce development timelines and regulatory uncertainty by providing analytical method development, process development, quality control (qc), and manufacturing services under one roof. as important, their analytical development and. With extensive experience in the analysis of innovative products and thanks to a continuous investment in new technologies and machinery, quality assistance can assist you in the development of your recombinant adeno associated viral vectors (raavs) products from early phases to commercialisation.
Viral Vectors 101 Viral Applications With ngs biotesting, pathoquest offers characterization, quality control and release of viral vectors & plasmids across the manufacturing journey. learn more. 3pbiovian offers comprehensive access to quality control analytics for both adenovirus and aav vector gmp batches, ensuring compliance with regulatory standards for human use. our services include compendial assays, sample specific validation, and assistance in developing and validating product specific analytical methods. Companies developing viral vectors should seek outsourcing partners that can help reduce development timelines and regulatory uncertainty by providing analytical method development, process development, quality control (qc), and manufacturing services under one roof. as important, their analytical development and. With extensive experience in the analysis of innovative products and thanks to a continuous investment in new technologies and machinery, quality assistance can assist you in the development of your recombinant adeno associated viral vectors (raavs) products from early phases to commercialisation.
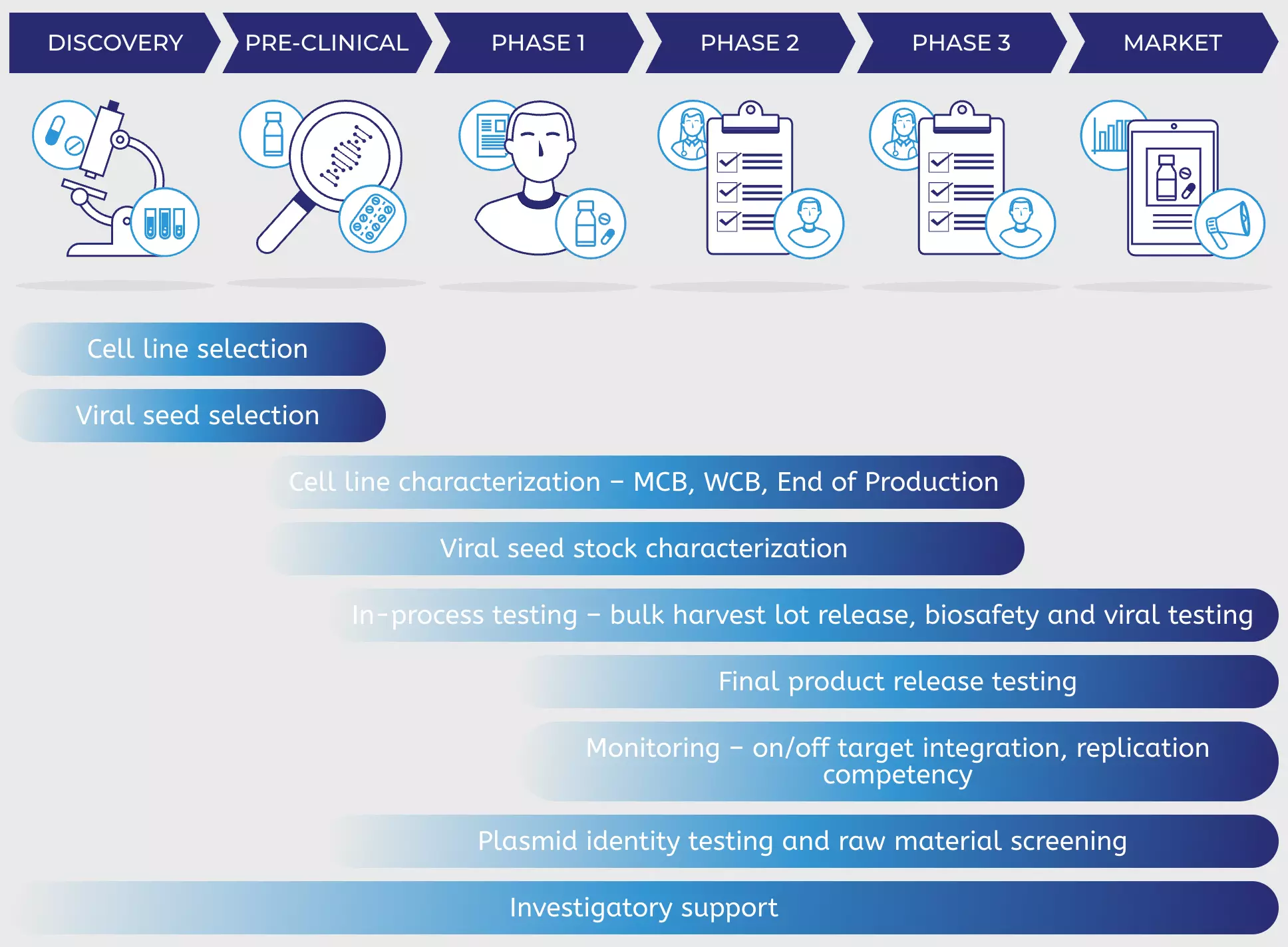
Quality Control Testing Of Viral Vectors Companies developing viral vectors should seek outsourcing partners that can help reduce development timelines and regulatory uncertainty by providing analytical method development, process development, quality control (qc), and manufacturing services under one roof. as important, their analytical development and. With extensive experience in the analysis of innovative products and thanks to a continuous investment in new technologies and machinery, quality assistance can assist you in the development of your recombinant adeno associated viral vectors (raavs) products from early phases to commercialisation.

New Equipment For Viral Vector Analysis Quality Assistance

Comments are closed.